Dictionary entry details
Origin of Name: From the Latin word cuprium, referring to the island of Cyprus: Date of Discovery: Known to the ancients: Discovered by: Copper beads dating back to 9000 B.C. Were found in Iraq.
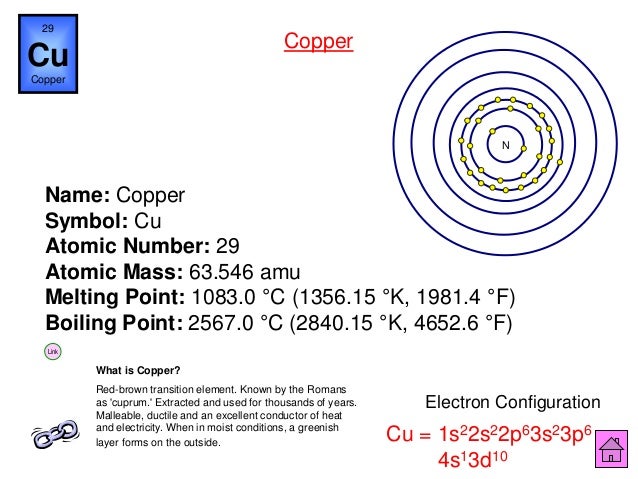
Atomic number of Cu = 29 The electronic configuration of Cu is, The reason for abnormal electronic configuration of Cu is because of the half-filled and fully-filled configuration are the more stable configuration. In this configuration, the d-subshell is fully-filled and s-subshell is half-filled which makes the Cu more stable. These subshells are made up of atomic orbitals. The four subshell labels that are used are s, p, d, and f. The maximum number of electrons allowed in each of these subshells are 2, 6, 10, and 14 respectively. For example, the names of the subshells in a sulfur atom would be 1s, 2s, 2p, 3s, and 3p (since sulfur has three electron shells). Would the phrase 'different mass number, same atomic number' be correct in describing an isotopes? 27 If a neutral copper atom (atomic number 29) becomes an ion that has a charge of 2+, how many electrons does the resulting ion have?
• ATOMIC NUMBER 29(noun)
Sense 1
Meaning:
A ductile malleable reddish-brown corrosion-resistant diamagnetic metallic element; occurs in various minerals but is the only metal that occurs abundantly in large masses; used as an electrical and thermal conductor

Classified under:
Nouns denoting substances
Synonyms:
atomic number 29; copper; Cu
Hypernyms ('atomic number 29' is a kind of...):
metal; metallic element (any of several chemical elements that are usually shiny solids that conduct heat or electricity and can be formed into sheets etc.)
conductor (a substance that readily conducts e.g. electricity and heat)
Hyponyms (each of the following is a kind of 'atomic number 29'):
blister copper (an impure form of copper having a black blistered surface)
Holonyms ('atomic number 29' is a substance of...):

bornite; peacock ore (a mineral consisting of sulfides of copper and iron that is found in copper deposits)
chalcocite; copper glance (a heavy grey mineral that is an ore of copper)
chalcopyrite; copper pyrites (a yellow copper ore (CuFeS2) made up of copper and iron sulfide)
cuprite (a mineral consisting of cuprous oxide that is a source of copper)
malachite (a green or blue mineral used as an ore of copper and for making ornamental objects)
brass (an alloy of copper and zinc)
bronze (an alloy of copper and tin and sometimes other elements; also any copper-base alloy containing other elements in place of tin)
Explanation is in a file
tinylnk.cf/8oKd
Explanation:
Answer is B.
B. It has a central nucleus composed of 29 protons and 35 neutrons,surrounded by an electron cloud containing 29 electrons.

I hope it's helpful!
Explanation:
Atomic mass is nuetrons + protons.
Basically it's
? nuetrons + protons = 64
I did process of elimination:
b) It cannot be 'b' because 29 + 64 doesn't equal 64.
c) It cannot be 'c' because in a regular molecule the amount of protons equals to amount of electron(s).
d) 29 + 29 doesn't = 64.
Therefore, the answer is 'A'
it's B
Copper Atomic Number 29
Explanation:
:p
The answer is B. It has a central nucleus composed of 29 protons and 35 neutrons, surrounded by an electron cloud containing 29 electrons.
Atomic Number 29 Element
is...
